Resources
Resources
In partnership with countries around the world, we have developed hundreds of resources to help strengthen the foundations of health systems. Please search our Resources to learn more about our publications, research, programmatic approaches, tools, and learnings.

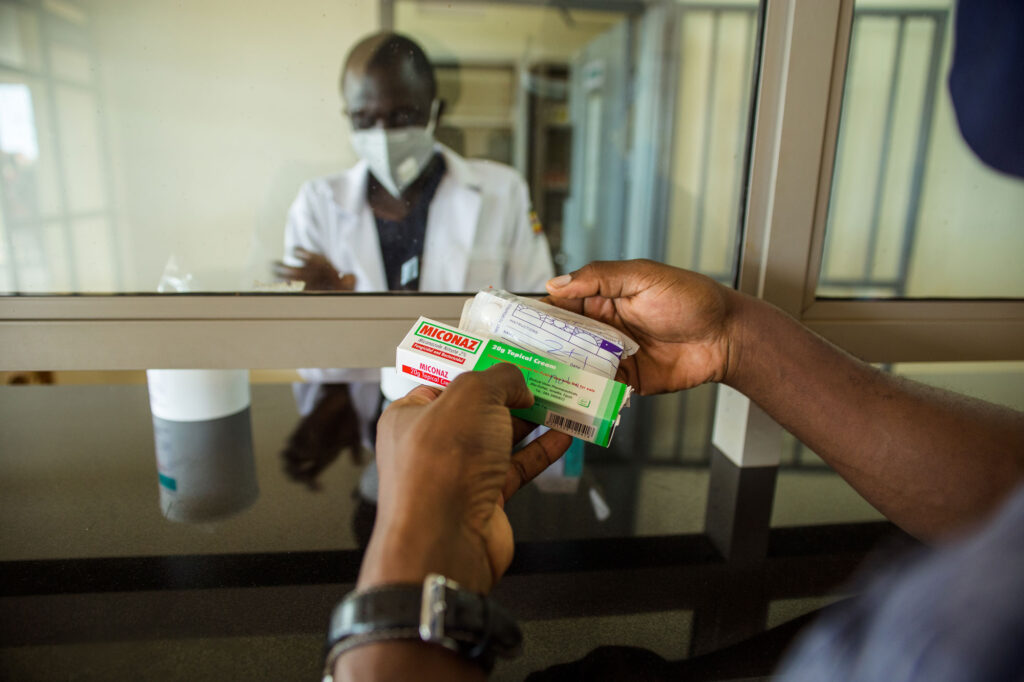
Strengthening Supply Chain and Pharmaceutical Systems for Sustained Health Impact
For more than three decades, Management Sciences for Health (MSH) has partnered with countries to build high-performing supply chains that ensure reliable access to safe, effective, quality-assured medicines and people-centered pharmaceutical services. MSH takes a holistic approach that fosters country-led innovation, whole-of-society engagement and collaboration, private-sector engagement, and effective leadership and governance. This ensures both reliable, affordable access to and sustainable delivery of medical products when and where they are needed and their appropriate use to save lives and improve health.
Topical
PHC Costing, Analysis, and Planning (PHC-CAP) Tool
Overview The PHC Costing, Analysis, and Planning (PHC-CAP) Tool helps program planners and decision makers increase coverage and improve the quality o…
Assessment of the Impact of Good Pharmacy Practices Training among Drug Dispensers in Bangladesh
Accelerating the End of TB: Field Research from Management Sciences for Health—2008-2022
A Collaborative on Contracting Organizations for Health-Related Services: Call for Expressions of Interest
USAID’s Health Systems for TB (HS4TB) Project is collaborating with the Joint Learning Network for Universal Health Coverage (JLN) to offer a Collabor…
Fact Sheet: NextGen Comprehensive Technical Assistance for Health Supply Chain and Pharmaceutical Management IDIQ
The MSH Consortium brings together dynamic organizations and leaders from the global and local supply chain and pharmaceutical fields, ready to create…
Uptake and Completion of Tuberculosis Preventive Treatment Using 12-Dose, Weekly Isoniazid–Rifapentine Regimen in Bangladesh: A Community-Based Implementation Study
Abstract This study assessed the uptake and completion of a community-based 12-dose, weekly isoniazid–rifapentine (3HP) TPT regimen in Dhaka, Banglade…
Enabling Cross-country Learning and Exchange to Support Universal Health Coverage Implementation
Abstract This paper emphasizes the need for country-led knowledge management in moving towards universal health coverage (UHC) and critiques tradition…

Overcoming Challenges in Private Sector Engagement for TB Control in Ethiopia: Experience from the USAID Eliminate TB Project

Improving Access to Patient-Centered Drug-Resistant Tuberculosis Management Services in Ethiopia

Strengthening Supply Chain and Pharmaceutical Systems for Sustained Health Impact

Request for Proposals: Management and Administration of Working Capital Loan Facility Guarantee Financing Mechanism
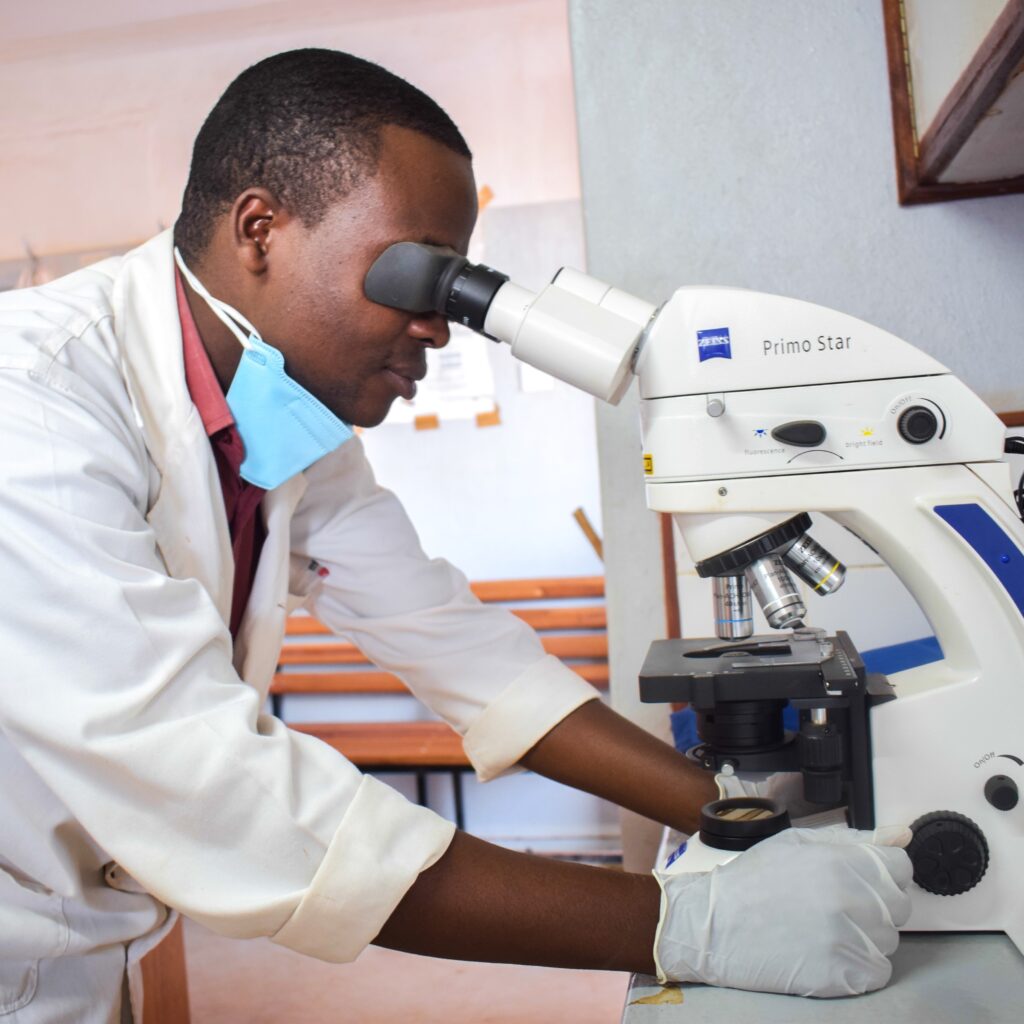
Why Laboratory Investment Matters: Rethinking the Role of Laboratory Services in Global Health