News & Stories
News & Stories
Management Sciences for Health, Beninese Government Continue Partnership with USAID to Strengthen Benin’s Health System
MSH will continue its longstanding partnership with the Government of Benin and USAID with a new project to unlock the potential of Benin’s own resources to improve its population’s health. MSH has assembled a strategic and primarily local consortium of partners and a women-led, all Beninese team to lead key areas, including expanding national health insurance, engaging private providers, and facilitating providers’ access to financial solutions.
Management Sciences for Health, Beninese Government Continue Partnership with USAID to Strengthen Benin’s Health System
MSH will continue its longstanding partnership with the Government of Benin and USAID with a new project to unlock the potential of Benin’s own resources to improve its population’s health. MSH has assembled a strategic and primarily local consortium of partners and a women-led, all Beninese team to lead key areas, including expanding national health insurance, engaging private providers, and facilitating providers’ access to financial solutions.
MSH at the People that Deliver Global Indaba 2024
Delving into the conference theme, The supply chain workforce: Solutions to transform health supply chains, our delegation shared insights on leadership, supply chain transformation, capacity building, partnerships, and workforce development, drawing on over three decades of MSH leadership in pharmaceutical and supply chain systems strengthening.

Beyond the Last Mile: Fighting Malaria with Spot Checks and Integrity

The Affordable Medicines Program Is Helping Ukraine Achieve Health Equity Despite the War

Using Treated Bed Nets to Fight Malaria in Nigeria’s Changing Climate Landscape
Management Sciences for Health, Beninese Government Continue Partnership with USAID to Strengthen Benin’s Health System
Arlington, VA—April 3, 2024—Management Sciences for Health (MSH) today announced it will continue its longstanding partnership with the Government of …
Management Sciences for Health Welcomes New Board Directors Magda Robalo and Ahmed Mushtaque Raza Chowdhury
Arlington, VA—March 25, 2024—Management Sciences for Health (MSH) is pleased to welcome two new members to its Board of Directors: Magda Robalo and Ah…

Charting a Path to TB Elimination: Financing and Governance Strategies for High-Burden Countries
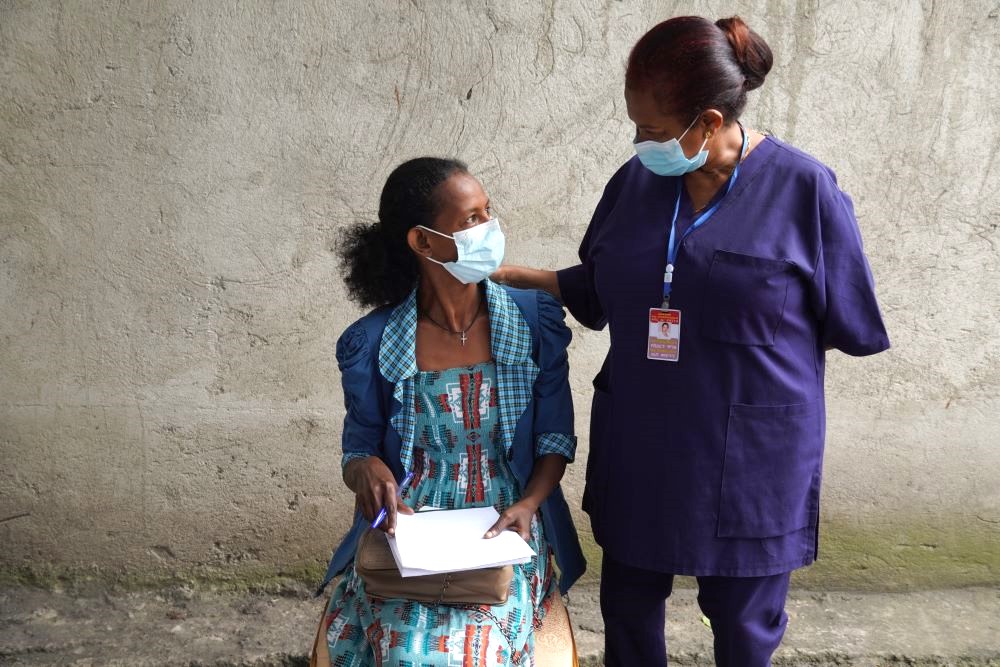
Eliminating TB in Ethiopia: One Woman’s Journey Back to Health at Adare General Hospital

Empowering Local Health Teams in Nigeria for Enhanced Governance in Malaria Programs

Listening to the People’s Voice: MSH Convenes Discussion around New Survey Results
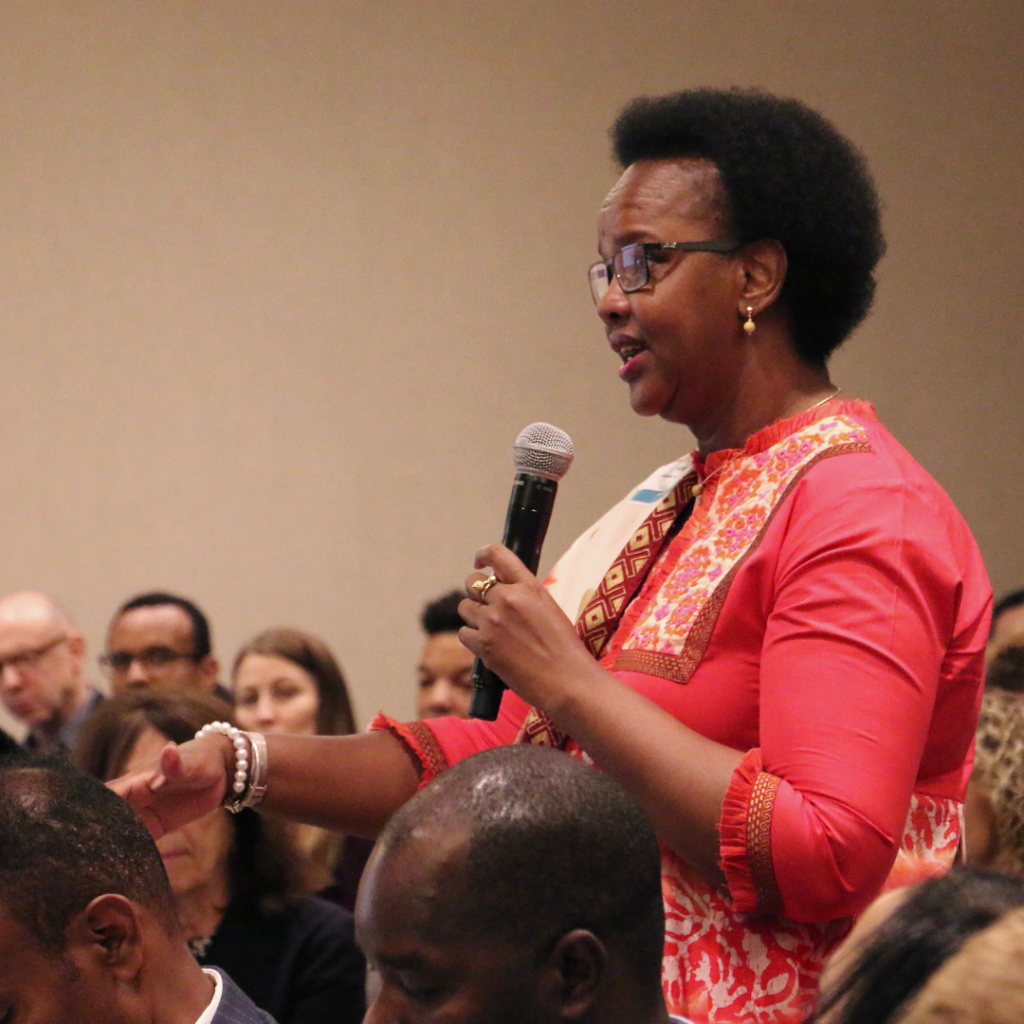
Expert Voices: Dr. Alaine Nyaruhirira on Strong Laboratories Systems
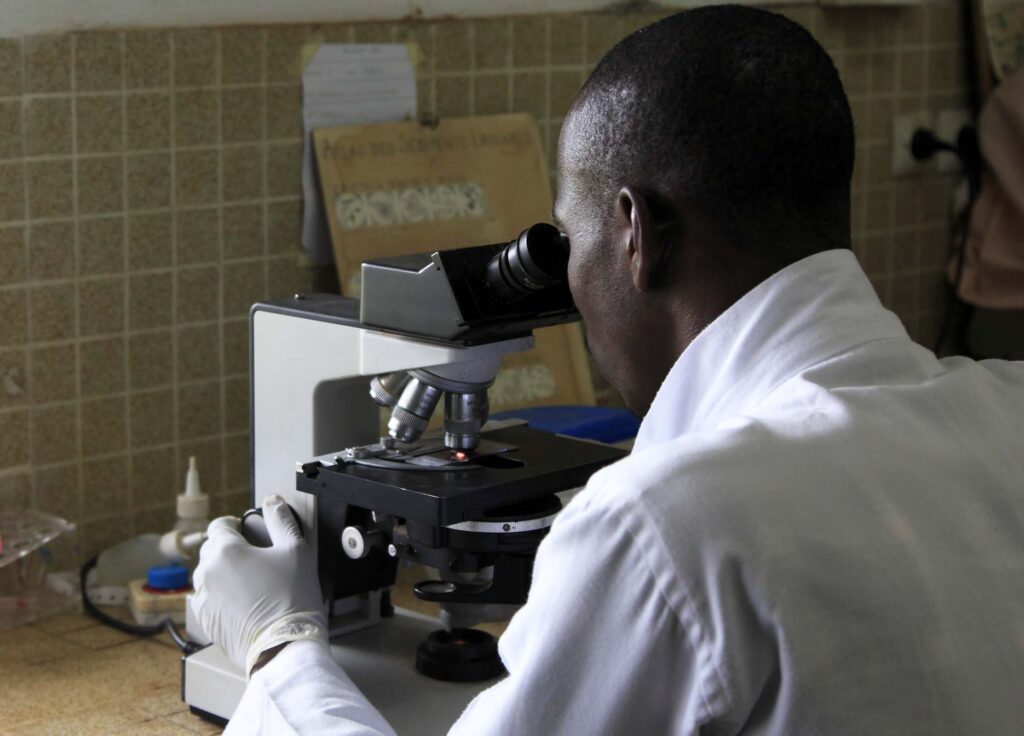
MSH Joins with Mott MacDonald, ICF to Take on Stubborn Diagnostic Gap Problem
